Legislative framework

Biocidal Products (BPs) are mixtures containing or generating one or more active substances, with the intention of destroying, deterring, rendering harmless, preventing the action of, or otherwise exerting a controlling effect on, any harmful organism by any means other than mere physical or mechanical action.
The aim is to protect humans and livestock, prevent damage to foods, consumer products, building materials (wood) and other products.
Regulation (EC) No. 528/2012 concerning the making available on the market and use of biocidal products, known also as the Biocidal Product Regulation (BPR), has been adopted with the purpose to improve the free movement of biocidal products within the Union while ensuring a high level of protection of both human and animal health and the environment.
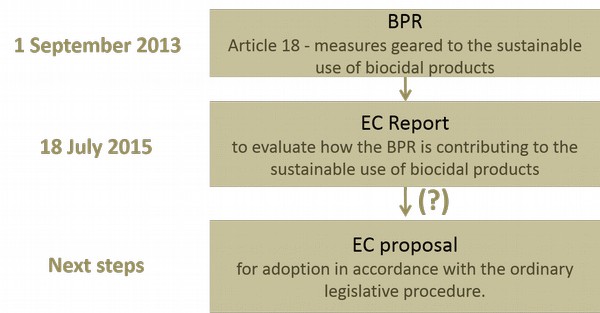
Reduction of the risks posed to human health, animal health and the environment by biocidal products shall be achieved through the sustainable use of biocidal products [Article 18 to BP]. The following measures could be considered:
- Define risks posed by the use of BPs in specific areas such as schools, workplaces, kindergardens, public spaces, geriatric care centres or in the vicinity of surface water or groundwater and identify measures to address those risks;
- Identify ways of improving performance of the equipment used for applying BPs;
- Promote best practices to reduce the use of BPs to a minimum;
- Identify most effective approaches for monitoring the use of BP;
- Develop & apply integrated pest management principles.
The time-frame for the sustainability of biocidal products is indicated in the following scheme:
This module is focused on the identification of hazards and risks posed to human health from the use of BPs in pest control activities, the BP labels and safety data sheets as hazard communication tools for workers and consumers of BPs, and indicative proposed measures to address these risks thereby ensuring safety at the workplace.
Hazard Identification & Characterization
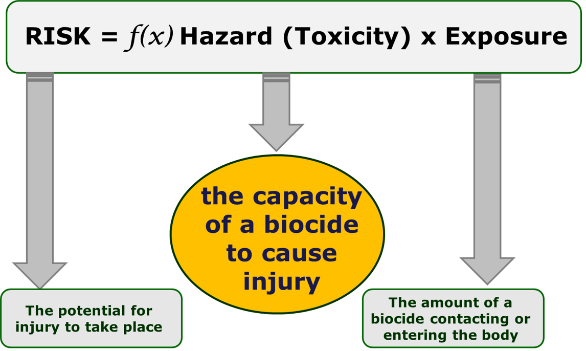
What are the health effects from biocide exposure?
Hazard is the inherent capacity of a biocide to cause injury (toxicity).
In practice, hazard is identified by specifically designed in vitro assays, in vivo mammalian toxicology studies and human epidemiological data (e.g. clinical cases from poisons units, records for manufacturing personnel etc), that overall aim to address acute toxicity, irritation, corrosivity, sensitization, repeated dose toxicity, mutagenicity, carcinogenicity and toxicity for reproduction.
Hazard may be further characterized via the setting of specific threshold values (ADI, ARfD, AELs) which represent the amounts of the biocide that are not anticipated to produce any adverse effects in humans.
Risk is the potential for injury to take place.
Routes of exposure to biocidal products

There are four ways in which BPs can enter the human body:
- through the skin (dermal),
- through the eyes,
- through the mouth (oral),
- through the lungs (respiratory or inhalation)
As part of the exposure assessment the concentrations/doses to which human populations (i.e. workers, consumers and man exposed indirectly via the environment) are or may be exposed should be estimated.
Risk characterisation
Risk characterisation is the estimation of the likelihood for the adverse effects to occur in a human population considering the actual or predicted exposure levels to a biocide.
Risk characterization may be qualitative or quantitative and may involve the following human population groups:
- Professional users (and industrial workers)
- Non-professional users (including the general public)
- Humans exposed via secondary pathways
In any case, anticipated human exposure levels to a biocide should be below the relevant hazard threshold value set for this biocide, in order to identify safe use(s).
How is risk characterised for a BP user?
Biocides used for Pest Control
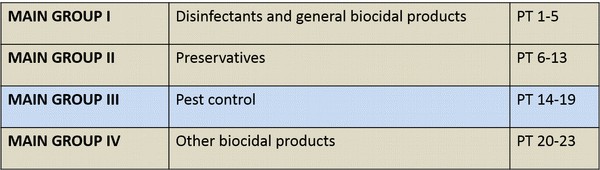
The following product types are used for pest control:
PT 14: Rodenticides (products used for the control of mice, rats or other rodents)
PT 15: Avicides (products used for the control of birds)
PT 16: Molluscicides (products used for the control of molluscs)
PT 17: Piscicides (products used for the control of fish; these products exclude products for the treatment of fish diseases)
PT18: Insecticides, acaricides & products to control other arthropods (products used for the control of arthropods (e.g. insects, arachnids and crustaceans))
PT 19: Repellents and attractants (products used to control harmful organisms (invertebrates such as fleas, vertebrates such as birds), by repelling or attracting, including those that are used for human or veterinary hygiene either directly or indirectly)
Level of toxicity to humans of biocides used for pest control
- GREAT variation with regard to level of toxicity to humans

Useful links
- REGULATION (EU) No 528/2012 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 May 2012 concerning the making available on the market and use of biocidal products (Text with EEA relevance) Download
- ECHA Guidance for Human Health Risk Assessment, Volume III, Part B: GUIDANCE ON REGULATION (EU) No 528/2012 CONCERNING THE MAKING AVAILABLE ON THE MARKET AND USE OF BIOCIDAL PRODUCTS (BPR). Version 1.0, December 2013 Download


